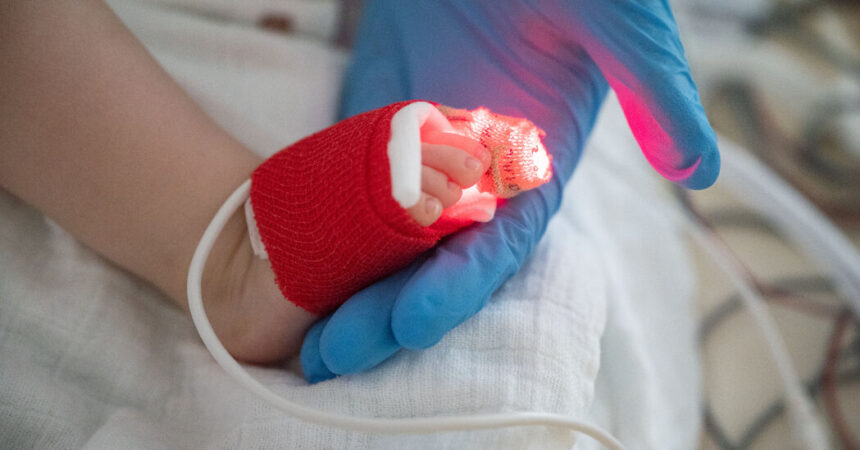
The Information
A Meals and Drug Administration advisory panel beneficial approval of a monoclonal antibody shot geared toward stopping a doubtlessly deadly pathogen, respiratory syncytial virus, or R.S.V., in infants and weak toddlers.
The remedy, referred to as Beyfortus by its builders Sanofi and AstraZeneca, can be the second such remedy that the F.D.A. has allowed to be given to very younger youngsters to stop R.S.V., which is a number one killer of infants and toddlers globally. An analogous remedy authorized greater than 20 years in the past is given in a number of doses and is just authorized for high-risk infants.
The 21-member panel voted unanimously in favor of giving the remedy to infants born throughout or getting into their first R.S.V. season. The advisers voted 19-2 for giving the shot to youngsters as much as 24 months of age who stay weak to extreme illness.
Why It Issues: R.S.V. is a worldwide killer of infants.
Although many individuals expertise this widespread virus as a routine chilly, it may be critical in younger infants and older adults. In keeping with the Facilities for Illness Management and Prevention, as much as 80,000 youngsters youthful than 5 are hospitalized with the virus annually and as much as 300 die. R.S.V. performed a task in filling youngsters’s hospitals throughout this winter’s “tripledemic,” which additionally included the flu and Covid-19.
For adults 65 and older, as many as 160,000 hospitalizations are attributed to R.S.V., and about 10,000 deaths. Vaccines for older adults have additionally not too long ago been authorized.
Background: The shot’s security might be monitored.
Greater than 3,200 infants got the antibody shot throughout research supplied to the F.D.A. by the drugmakers, together with one which discovered that after six months, efficacy towards very extreme R.S.V. that required medical consideration was 79 %.
A separate company panel has beneficial approval of a maternal R.S.V. vaccine that can also be beneath evaluate. A few of the advisers raised issues about knowledge for that vaccine, and for one more prefer it that advised a small improve in preterm births.
If the antibody remedy is authorized, the F.D.A. stated it might proceed to watch the remedy for security utilizing a number of knowledge sources. AstraZeneca stated it might additionally conduct periodic security evaluations utilizing worldwide knowledge.
What’s Subsequent: The C.D.C. will evaluate pictures for moms and infants.
If the company approves the brand new shot, it’ll doubtless turn into obtainable within the fall — across the identical time that the Pfizer R.S.V. vaccine given throughout being pregnant referred to as Abrysvo goes available on the market.
The C.D.C. is anticipated to advise well being suppliers on the usage of the brand new therapies later this month. Households and their docs may then select a course of remedy that will bear in mind the timing of a delivery and the winter R.S.V. season, amongst different components.
The F.D.A. stated there was no research of the dangers or advantages of ladies taking the maternal R.S.V. vaccine and giving the antibody pictures to their infants.