Blood pattern for respiratory syncytial virus (RSV) take a look at
Jarun011 | Istock | Getty Photographs
The Meals and Drug Administration on Monday accepted AstraZeneca and Sanofi‘s shot that protects infants and toddlers towards respiratory syncytial virus, which is the main explanation for hospitalization amongst infants within the U.S.
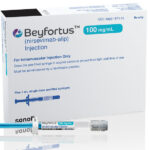
Nirsevimab is the primary shot accepted by the FDA to guard all infants towards RSV no matter whether or not they’re wholesome or have a medical situation.
associated investing information

The FDA approval of nirsevimab, offered below the model title Beyfortus, comes forward of RSV season this fall. The Facilities for Illness Management and Prevention’s panel of impartial specialists will meet in August to make suggestions on how the shot needs to be administered by docs.
One other shot referred to as palivizumab is already in the marketplace, however it’s given primarily to infants who’re preterm or who’ve lung and congenital coronary heart circumstances that put them at excessive danger of extreme illness. Nirsevimab, is run as a single injection. It is a main benefit over palivizumab, which is run month-to-month all through the RSV season.
Nirsevimab is run both earlier than or throughout an toddler’s first RSV season. Toddlers as much as two years outdated who stay susceptible may also obtain the shot throughout their second RSV season.
RSV is a significant public well being risk that kills practically 100 infants yearly, in line with a research printed within the medical journal JAMA Open Community final 12 months. The virus is the main explanation for hospitalization amongst youngsters lower than a 12 months outdated, in line with a research printed within the Journal of Infectious Illnesses.
A surge in RSV infections final fall overwhelmed youngsters’s hospitals throughout the U.S. and led to requires the Biden administration to declare a public well being emergency in response.
Nirsevimab was as much as 75% efficient at stopping decrease respiratory tract infections that required medical consideration amongst infants, and 78% efficient at stopping hospitalization, in line with a FDA evaluate.
The FDA didn’t recognized any security issues in its evaluate of nirsevimab, although different monoclonal antibodies have been related to allergic reactions reminiscent of pores and skin rashes.
Nirsevimab is a monoclonal antibody that has an analogous perform to a vaccine. Vaccines stimulate the immune system to supply protecting antibodies, whereas photographs like nirsevimab ship these antibodies straight into the bloodstream.
The truth that nirsevimab is regulated as a drug has created some uncertainty about whether or not the federal Vaccines for Kids program will present the shot free of charge to households who face monetary difficulties. The CDC advisors are anticipated to debate this problem at their August assembly.
Households might need two choices to guard their infants this fall. Pfizer has developed a vaccine that protects infants by administering the shot to the mom whereas she is pregnant. The FDA’s impartial advisors really helpful Pfizer’s vaccine in Might. The company is predicted to make a last choice on whether or not to approve the shot in August.