Why It Issues: A illness with a toll that’s tough to think about.
An estimated 100,000 folks in the USA have sickle cell illness, most of whom have African ancestry. Sickle cell shortens lives, injures organs and bones and causes episodes of searing ache that may repeatedly ship sufferers to emergency rooms, or result in prolonged hospital stays.
A report by the Institute for Medical and Financial Overview stated that for individuals who don’t have sickle cell illness, “it’s arduous to grasp the bodily, emotional and psychological toll.” Folks with the illness, the report added, “not solely described intense fatigue, nervousness and melancholy, however at occasions excessive hopelessness.”
One affected person, Mariah Jacqueline Scott, 32, who lives in Highland Park, N.J., has had two hip replacements, two shoulder replacements, a splenectomy, a gall bladder removing and a tonsillectomy due to the illness. She spent the yr after her daughter was born out and in of the hospital being handled for excessive ache attributable to blocked blood vessels. She had her second shoulder alternative after her shoulder collapsed whereas she was holding her child.
The one remedy has been a bone-marrow transplant, which requires discovering a donor, present process intensive chemotherapy and taking immunosuppressive medicine. However gene modifying gives another. Vertex and CRISPR Therapeutics, the makers of the remedy being taken up by the F.D.A. committee on Tuesday, stated that in scientific trials, signs of the illness went away after sufferers had the remedy. Thus far, the sufferers look like cured. The approach prompts a gene that may make usually functioning blood cells.
Ms. Scott stated she knew gene modifying was arduous, however she was critically contemplating present process the remedy when it grew to become accessible.
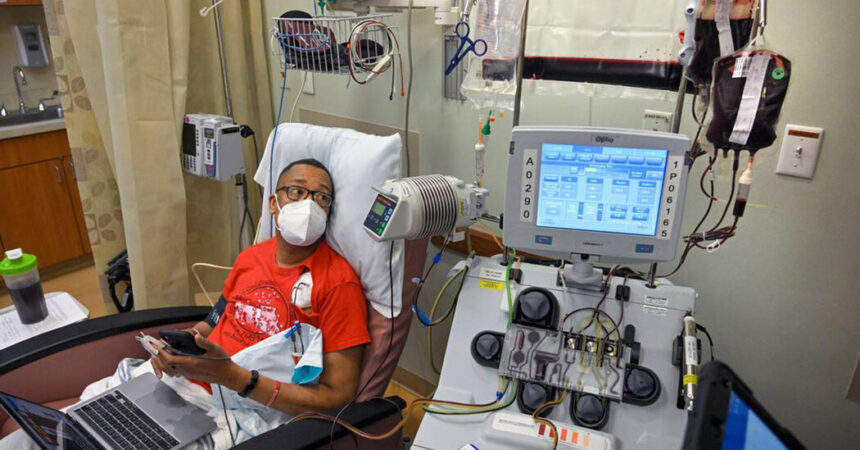
Details to Preserve in Thoughts: Gene therapies carry their very own challenges.
Vertex’s remedy begins when docs take away stem cells from the blood and ship them for remedy. Subsequent comes intense chemotherapy to fully clear the bone marrow earlier than the handled cells are injected. After that, sufferers should spend at the very least a month in a hospital whereas the handled cells repopulate the bone marrow.
As a result of every affected person’s cells should be handled individually there are questions on how rapidly firms can ramp up manufacturing.
“Manufacturing may be very sophisticated,” stated Dr. Stephan Grupp, chief of the mobile remedy and transplant part of Kids’s Hospital of Philadelphia, who consults for Vertex.
Therapy will likely be extraordinarily costly, probably within the hundreds of thousands of {dollars} per affected person, and the businesses won’t say what number of sufferers they count on to have the ability to deal with at first.
Gene modifying also can impose private hardship on sufferers and their households. A hospital with the experience to manage the remedy and look after sufferers could also be removed from residence. And sufferers should keep there for an extended time frame.
What’s subsequent: Extra F.D.A. choices and extra medicine.
If the advisory committee recommends the Vertex remedy, the F.D.A. will determine whether or not to approve it on Dec. 8.
On Dec. 20, the F.D.A. will determine on one other software for sickle cell gene remedy made by Bluebird Bio. Two different firms and a tutorial heart, Boston Kids’s Hospital, are testing their very own sickle cell gene therapies.
Whereas these therapies may scale back the struggling of sickle cell sufferers in the USA and different rich nations, there’s a fair larger want for them in some growing nations like Nigeria. Nevertheless, they are going to be tough to export to growing nations as a result of the therapies are extraordinarily costly and so they can solely be administered at hospitals the place docs have experience in quite a few superior strategies.
One firm, Beam, is testing a means to offer gene modifying that requires nothing greater than a single infusion in a physician’s workplace. Vertex has what it calls an “aspirational” technique that might ship gene modifying in a capsule.